Article 4
The Promise of Engineered Skeletal Muscles for Drug Discovery
Roshini Beenukumar, PhD
Age-associated muscle function decline presents significant challenges in older adults. To develop effective therapeutics, there is a need for more representative human skeletal muscle models, especially for drug discovery. Recent advancements in engineered skeletal muscle tissues and human-induced pluripotent stem cell (hiPSC)-derived muscle cells show promise. Yet, challenges persist in achieving mature, functional muscle cell phenotypes in vitro. Here, we discuss novel approaches to culturing functional skeletal muscle tissue for drug discovery and disease modeling applications.
Muscle function decline is one of the many stark realities of aging. Sarcopenia—the gradual decline in skeletal muscle mass and function—leads to decreased strength and power and, consequently, a higher incidence of falls and fractures (1).
Various physiological mechanisms contribute to the development of sarcopenia, including denervation leading to the loss of motor units and muscle fibers, reduced production of anabolic hormones like testosterone, growth hormone, and insulin-like growth factor-1, and increased release of catabolic agents such as interleukin-6(2).
Is age-associated sarcopenia reversible? A recent study led by Professor Yuji Yamanashi of the Institute of Medical Science at the University of Tokyo shows promise. Using a gene therapy approach, the team showed that improving muscle innervation at the neuromuscular junction enhances motor function and muscle strength in aged mice (3).
While animal models, as used in the above study, help gain significant insights into the underlying genetic, physiological, and biochemical mechanisms involved in muscle function, they often fail to replicate the complexity seen in humans for drug discovery applications.
Engineered skeletal muscle tissues for drug discovery
Engineered skeletal muscle tissues that mimic the complexity of the in vivo environment are revolutionizing how diseases are modeled, and treatments are tested. These in vitro models can be a more relevant and accurate platform for drug testing than traditional animal models and 2D cell culture (4).
Recent studies have shown that cell sheet-based engineering can produce human muscle tissues with sufficient contractile properties, which is critical in drug testing scenarios (5).
Another significant milestone was the development of human bio-artificial muscle through a seven-day tissue engineering procedure. This process involves the fusion and differentiation of human myoblasts into aligned myofibers within an extracellular matrix, providing a more physiologically relevant model for drug discovery and disease modeling (6).
Another significant milestone was the development of human bio-artificial muscle through a seven-day tissue engineering procedure. This process involves the fusion and differentiation of human myoblasts into aligned myofibers within an extracellular matrix, providing a more physiologically relevant model for drug discovery and disease modeling (6).
hiPSC-derived muscle cells as drug discovery models for muscular dystrophies
The application of engineered skeletal tissues in disease modeling of muscular dystrophies has gained attention in recent years. Muscular dystrophies are a diverse group of muscle disorders characterized by progressive weakness and degeneration of skeletal muscles (7).
One of the most common forms of muscular dystrophy, Duchenne Muscular Dystrophy (DMD), has been a focus of such studies. DMD is characterized by a mutation in the dystrophin gene, leading to progressive muscle degeneration and weakness, and eventually death due to cardiac and respiratory complications. Although treatments like glucocorticosteroid and exon-skipping therapy exist, their efficacy is limited or uncertain (8).
A recent study showed that DMD patient-derived hiPSCs when differentiated into myoblasts, display a DMD-relevant phenotype, specifically myogenic fusion deficiency. Using these differentiated myoblasts, the authors developed a drug screening platform to identify potential treatments for DMD. The identified compounds—ginsenoside Rd and fenofibrate—showed promise in preclinical tests on mdx mice, a model for DMD, ameliorating some skeletal muscle phenotypes caused by dystrophin deficiency (8).
Challenges with in vitro models of skeletal muscle cells
While hiPSC-derived muscle cells provide a promising platform for drug discovery in muscular dystrophies, challenges related to cell maturation remain significant barriers. Moreover, due to the developmental late-onset nature of several muscular dystrophies, immature cell-based models may only partially replicate the adult disease phenotype. Therefore, methods that promote the maturation of cultured skeletal muscle cells must be employed to resemble native architecture and function (7).
Several strategies can be applied to mature skeletal muscle cells in vitro. Creating a microenvironment that mimics in vivo conditions is crucial. Aging, or extending culture duration, has proven effective in enhancing maturation. Substrate elasticity plays a pivotal role in promoting natural growth patterns, as does the alignment and three-dimensionality of the cells. Electromechanical conditioning that mimics exercise has also been shown to advance maturation. Additionally, growth factor supplementation and co-culturing with supportive cell types like motor neurons and endothelial cells aid in achieving a mature phenotype, though managing complex cultures presents challenges (7).
Cell-sheet technology for functional in vitro muscle tissue models
Cell-sheet technology has shown promise in producing engineered human muscle tissues with contractile properties. It involves using a thermo-responsive micropatterned substrate for aligning myofiber structures, which are harvested as single-cell sheets. These sheets are layered on a fibrin-based gel, fostering myofiber maturation and providing an elastic platform for contraction. The engineered muscle tissue exhibits high contractile force and unidirectional contraction in response to electrical and chemical stimuli. Additionally, it allows real-time quantitative assessment of physiological responses to drugs, indicating its potential as a viable tissue model for muscle physiology and drug discovery research (5).
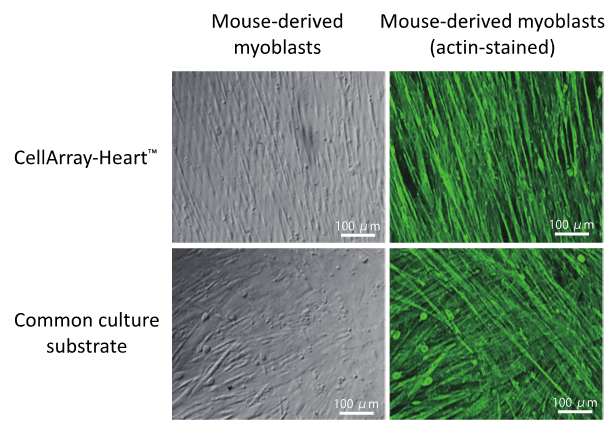
Figure 1: Unidirectional orientation of mouse-derived myoblasts (C2C12 strain)
cultured on CellArray-Heart ™.
When compared to a common cell culture substrate, CellArray-Heart™ produced unidirectional cell sheets as shown by actin immunostaining.
cultured on CellArray-Heart ™.
When compared to a common cell culture substrate, CellArray-Heart™ produced unidirectional cell sheets as shown by actin immunostaining.

At OJI Holdings, we have established a new cell culture substrate – CellArray-Heart™ – to produce single flat unidirectional cell sheets (see Figure 1). CellArray-Heart™ features a surface microstructure with nano-sized pillars and flat areas arranged in stripes that orient various cell types, including myoblasts, cardiomyocytes, and fibroblasts, in one direction during regular cell culture.
References
1. Cruz-Jentoft, A. J. et al. (2010). Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing, 39(4), 412–423.
2. Deschenes M. R. (2004). Effects of aging on muscle fibre type and size. Sports medicine (Auckland, N.Z.), 34(12), 809–824.
3. Ueta, R. et al. (2020). DOK7 gene therapy enhances neuromuscular junction innervation and motor function in aged mice. iScience, 23(8),101385.
4. Ostrovidov, S. et al. (2023). Latest developments in engineered skeletal muscle tissues for drug discovery and development. Expert opinion on drug discovery, 18(1), 47–63.
5. Takahashi, H., Wakayama, H., Nagase, K., & Shimizu, T. (2023). Engineered human muscle tissue from multilayered aligned myofiber sheets for studies of muscle physiology and predicting drug response. Small methods, 7(2), e2200849.
6. Gholobova, D. et al. (2018). Human tissue-engineered skeletal muscle: a novel 3D in vitro model for drug disposition and toxicity after intramuscular injection. Scientific reports, 8(1), 12206.
7. Smith, A. S. T., Davis, J., Lee, G., Mack, D. L., & Kim, D. H. (2016). Muscular dystrophy in a dish: engineered human skeletal muscle mimetics for disease modeling and drug discovery. Drug discovery today, 21(9), 1387–1398.
8. Sun, C. et al. (2020). Duchenne muscular dystrophy hiPSC-derived myoblast drug screen identifies compounds that ameliorate disease in mdx mice. JCI insight, 5(11), e134287.