Article 2
Can adult-like iPSc cardiomyocytes
improve cardiotoxicity screening?
improve cardiotoxicity screening?
Roshini Beenukumar, PhD
Cardiotoxicity is a significant concern throughout the drug development process, from preclinical and clinical phases to post-market surveillance. While animal models are commonly used to assess cardiotoxicity, they often fail to accurately predict toxicity in humans. As a result, novel assays based on human induced pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) are being integrated into cardiotoxicity screening protocols. In this article, we explore the benefits and obstacles of utilizing human iPSC-CMs for cardiotoxicity screening and present a new cell culture substrate that promotes iPSC-CM maturation.
During drug development, failure at clinical trials is a costly affair. Almost half of drug failures occur due to safety concerns that go undetected during animal testing or preclinical phases (1). One particular area of concern is cardiotoxicity – a common reason drugs are withdrawn from the market due to adverse drug reactions (2,3). The limitations of existing methods for detecting cardiotoxicity in investigational drugs highlight the need for more accurate and comprehensive models for cardiotoxicity screening during the drug development process (4).
Among the various methods for preclinical cardiotoxicity screening, animal models are still the most widely used. However, they fail to fully replicate human physiology and metabolism, which can lead to inaccurate predictions of toxicity in humans. Additionally, there are ethical concerns about the use of animals, particularly larger animals, in testing. Hence, there has been a push to integrate novel methods such as in silico modeling, which use computational simulations to model the human heart and its response to drugs, and iPSC- or primary cardiomyocyte-based assays for cardiotoxicity screening (4).
Human iPSC-CMs, owing to their easy accessibility and amenability to genetic engineering, are being embraced by the research community as well as regulatory authorities as a promising addition to cardiotoxicity screening protocols. The Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative, which includes members of the FDA, EMA, Japan NIHS, PMDA, and other global regulatory agencies, recommends human iPSC-CM assays to confirm findings of in vitro and in silico assays as part of their comprehensive in vitro proarrhythmic assay protocol (5).
Very recently, the US FDA announced that animal testing is no longer required before human trials, welcoming alternative methods for preclinical drug testing (6). This move comes after the Wellcome Sanger Institute in the UK announced in 2019 that it will be closing its animal research facility, one of the most well-known centers in the world (7). These historic announcements are indicative of the wider global movement to replace animal testing with potential substitutes like iPSCs and organoids.
Here are some of the advantages offered by hiPSC-CMs for drug-toxicity screening (8):
• Scalability: iPSCs can be easily scaled, allowing for the efficient and sustainable production of cardiomyocytes for large-scale drug screening.
• Patient-specificity: hiPSC-CMs can facilitate precision medicine approaches as it allows the correlation of a patient’s clinical/genetic information with their in vitro pharmacological responses.
• Disease-specificity: Disease-specific models of hiPSC-CMs have the potential to serve as platforms for studying disease mechanisms and testing new therapeutics.
• Patient stratification: hiPSC-CMs derived from a representative pool of the general population could enable the stratification of patients for clinical trials.
• Patient-specificity: hiPSC-CMs can facilitate precision medicine approaches as it allows the correlation of a patient’s clinical/genetic information with their in vitro pharmacological responses.
• Disease-specificity: Disease-specific models of hiPSC-CMs have the potential to serve as platforms for studying disease mechanisms and testing new therapeutics.
• Patient stratification: hiPSC-CMs derived from a representative pool of the general population could enable the stratification of patients for clinical trials.
Human iPSC-CMs to study chemotherapy-induced cardiotoxicity in cancer
Cardiotoxicity induced by cancer therapeutics, including anthracyclines, anti-microtubule agents, tyrosine kinase inhibitors (TKIs), and antibody-based drugs, remains a significant concern in cancer drug development (9). iPSC-CMs, particularly those derived by differentiating individual patient cells, can potentially add to or replace animal testing for cancer therapy-induced cardiotoxicity. For example, doxorubicin, a chemotherapy agent commonly used alone or in combination with other drugs to treat a variety of cancers, is known to cause cardiotoxicity. Several studies have successfully used iPSC-CMs to elucidate the mechanisms of doxorubicin-induced cardiotoxicity (10). In one study, doxorubicin-treated iPSC-CMs were used to evaluate global transcriptome changes in response to changes in exposure time and dose (11). In another study, using iPSC-CMs from breast cancer patients, the authors showed doxorubicin increases cellular ROS production, calcium handling, whole-cell oxidative stress, and ultimately double-stranded DNA damage (12).
Although promising, employing iPSC-CMs for drug toxicity assessment has a number of drawbacks. For instance, disparities exist between iPSC-CM assays and clinical experience. (13). Another major challenge is their immaturity which makes it difficult to accurately assess the response of the chemotherapeutic agents tested. To overcome these challenges and improve time and cost-efficiency, new and improved protocols for iPSC-based drug toxicity screening has to be developed in the near future (9).
Achieving maturation is critical to using human iPSC-CMs in drug testing
The immaturity of the human iPSC-CMs are evident in their structural and functional limitations such as their abnormal spontaneous beating pattern, less hyperpolarized resting membrane potential, slower upstroke velocity, and shorter action potentials, which limit their use in preclinical cardiotoxicity testing (4). To address these limitations, various strategies have been developed to promote the maturation of iPSC-CMs, such as long-term cell culture, electric stimulation, mechanical stress, biochemical induction, and 3D tissue engineering using co-culture and patterned substrates. These methods aim to mimic the in vivo microenvironment and promote the maturation of the cells, making them more suitable for drug testing and research (14).
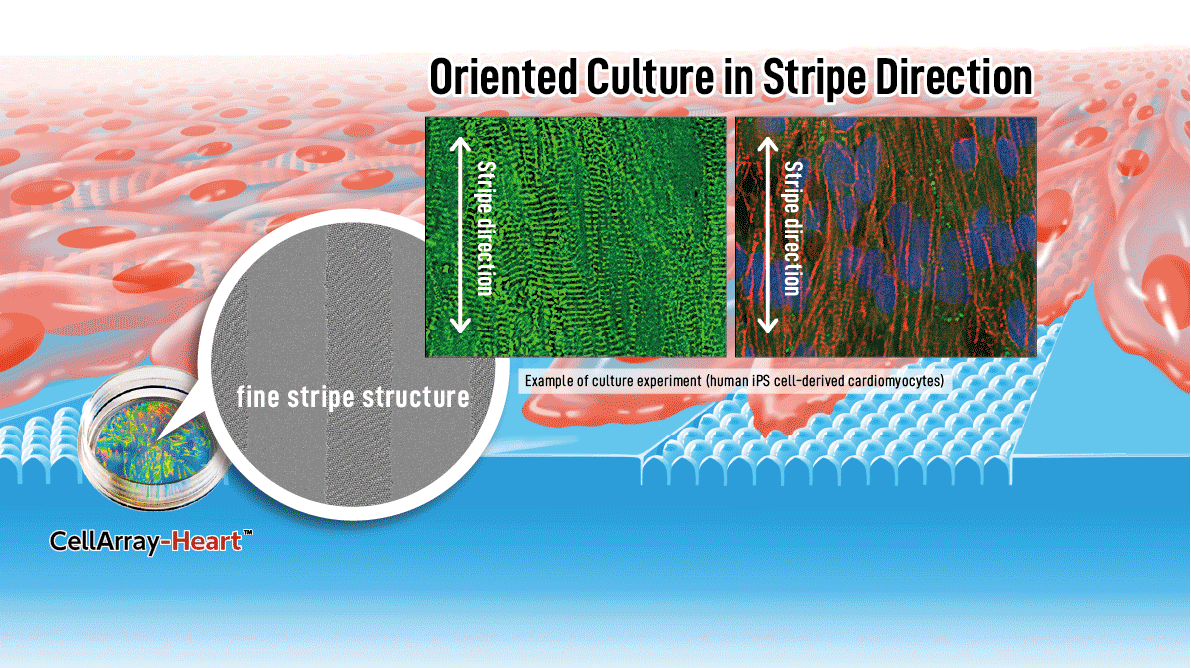
A novel “fine stripe structure” cell culture substrate for human iPSC-CM maturation
The native myocardium comprises multiple layers of aligned cardiac tissue; therefore, controlling the alignment of cardiomyocytes is crucial for optimal tissue function (15). One essential tool for promoting alignment, and thereby maturation, is the patterning of the culture substrate. However, currently available substrates such as nanofiber scaffolds and those with simple groove structures are not conducive to cell-cell coordination and alignment. To address these limitations, we have developed a new cell culture substrate, CellArray-Heart™, which enables oriented culture of iPSC-CMs by simply seeding cells onto the substrate. CellArray-Heart™ features a surface microstructure with nano-sized pillars and flat areas arranged in stripes, which vary the strengths of cell adhesion and orient iPSC-CMs in one direction to produce single flat unidirectional cell sheets.

References
1. Harrison R. K. (2016). Phase II and phase III failures: 2013-2015. Nature reviews. Drug discovery, 15(12), 817–818.
2. Onakpoya, I. J., Heneghan, C. J., & Aronson, J. K. (2016). Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC medicine, 14, 10.
3. Kocadal, K., Saygi, S., Alkas, F. B., & Sardas, S. (2018). Drug-associated cardiovascular risks: A retrospective evaluation of withdrawn drugs. Northern clinics of Istanbul, 6(2), 196–202.
4. Stella Stoter, A. M., Hirt, M. N., Stenzig, J., & Weinberger, F. (2020). Assessment of cardiotoxicity with stem cell-based strategies. Clinical therapeutics, 42(10), 1892–1910.
5. CiPA webpage: https://cipaproject.org/about-cipa/
7. Nature new article: https://www.nature.com/articles/d41586-019-01685-7
8. Pang, L. et al. (2019). Workshop report: FDA workshop on improving cardiotoxicity assessment with human-relevant platforms. Circulation research, 125(9), 855–867.
9. Sayed, N., Ameen, M., & Wu, J. C. (2019). Personalized medicine in cardio-oncology: The role of induced pluripotent stem cell. Cardiovascular research, 115(5), 949–959.
10. Huang, M. F., Pang, L. K., Chen, Y. H., Zhao, R., & Lee, D. F. (2021). Cardiotoxicity of Antineoplastic Therapies and Applications of Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cells, 10(11), 2823.
11. Chaudhari, U. et al. (2016). Identification of genomic biomarkers for anthracycline-induced cardiotoxicity in human iPSC-derived cardiomyocytes: An in vitro repeated exposure toxicity approach for safety assessment. Archives of toxicology, 90(11), 2763–2777.
12. Burridge, P. W. et al. (2016). Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nature medicine, 22(5), 547–556.
13. Blinova, K. et al. (2017). Comprehensive translational assessment of human-induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicological sciences: an official journal of the Society of Toxicology, 155(1), 234–247.
14. Ahmed, R. E., Anzai, T., Chanthra, N., & Uosaki, H. (2020). A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Frontiers in cell and developmental biology, 8, 178.
15. Takada, T. et al. (2022). Aligned human induced pluripotent stem cell-derived cardiac tissue improves contractile properties through promoting unidirectional and synchronous cardiomyocyte contraction. Biomaterials, 281, 121351.