HOME > Column > Article3:Can “maturing” human pluripotent stem cell-derived cardiomyocytes accelerate their adoption in regenerative medicine?
Article 3
Can “maturing” human pluripotent stem cell-derived cardiomyocytes accelerate their adoption in regenerative medicine?
Roshini Beenukumar, PhD
Cardiovascular diseases (CVDs) claim around 17.9 million lives each year. Despite being the leading cause of death worldwide, current treatments do not aim to reverse the damages caused by them. Stem cell-based regenerative therapies, such as human induced pluripotent stem cells (iPSCs), have the potential for myocardial restoration. In this article, we discuss the opportunities and challenges facing the adoption of iPSC cardiomyocytes (CMs) in cardiac regeneration. We also discuss a novel approach based on cell sheet technology to promote the maturation of iPSC-CMs, a critical obstacle delaying their application in regenerative medicine.
CVDs are becoming increasingly prevalent. This rise can be attributed to an aging population and medical advances that have increased the longevity of patients with CVDs. As the leading cause of death worldwide, CVDs claim around 17.9 million lives each year (1). The most prevalent CVD is acute myocardial infarction (AMI), causing nearly 3 million deaths each year. Around 5–10% of AMI survivors die within the first year, and roughly half require hospitalization within the same year (2).
AMIs cause irreversible damage to the heart muscle, can weaken diastolic and systolic functions, and make the patient susceptible to arrhythmias (2). The standard treatment options for AMI patients are percutaneous angioplasty and coronary artery bypass surgery. However, these therapies aim only to recover blood flow to the heart and not regenerate the injured myocardium. Therefore, alternative therapeutic strategies, particularly for patients suffering from severe heart disease, are needed to fill this unmet need (3).
Regenerative medicine approaches show promise in myocardial restoration
A comprehensive systematic review and meta-analysis of stem cell trials for heart failure (which included 31 included randomized controlled trials and 1521 participants) compared various cell therapies with placebo or control. The study concluded that cell therapies significantly reduced mortality and rehospitalization caused by worsening heart failure, moderately improved left ventricular systolic function, and improved heart failure symptoms, exercise capacity, and quality of life (4).
Despite this positive outlook, poor survival rates of various stem cells after direct injection into ischemic hearts impede their clinical adoption. More than 70% of cells die within the first two days after direct injection, and the surviving cells are progressively lost within the next several days (3).
Human induced pluripotent stem cells in cardiac regeneration – the case for maturation
Pluripotent stem cells, such as embryonic and induced pluripotent stem cells, are particularly advantageous in cardiac regeneration for various reasons. Their limitless regenerative capacity and ability to differentiate into any cell type give them the ability to compensate for the damage caused by AMI. In addition, iPSCs do not have the ethical and immunogenic concerns of embryonic stem cells and can be banked, thereby expanding clinical utility (3). Therefore, iPSCs are a favorable alternative to embryonic stem cells for regenerative therapy applications.
However, iPSCs are not without limitations. To be safe, iPSC-based technologies require methods of iPSC generation and controlled differentiation that minimize potentially deleterious genomic alterations. Another disadvantage, particularly emphasized in iPSC cardiomyocytes (iPSC-CMs), is their structural and functional immaturity. Phenotypically, iPSC-CMs are shorter, rounder, and thinner than adult cardiomyocytes. In addition, they lack transverse tubules, have a less well-developed sarcoplasmic reticulum, and are more likely to be mononuclear. In terms of metabolism and function, iPSC-CMs resemble fetal CMs. Due to fewer mitochondria, they use glycolysis instead of β-oxidation of fatty acids for energy. They also have higher resting membrane potentials and slower depolarization rates than adult ventricular cardiomyocytes. Furthermore, the transcription profile of iPSC-CMs resembles fetal rather than adult cardiomyocytes (5).
Cell sheet technology for iPSC-CM maturation
Various strategies have been developed to promote the maturation of iPSC-CMs. These methods, which include long-term cell culture, biochemical induction, mechanical stress, electric stimulation, and 3D tissue engineering using co-culture and patterned substrates, aim to mimic the in vivo microenvironment, improving their suitability for regenerative medicine applications (6).
Cell sheet technology is an increasingly popular approach for iPSC-CM maturation. Cell sheets generated without scaffold support eliminate the risk of inducing inflammatory reactions and promote cell survival (3). In one study, scaffold-free human cardiac tissue patches derived from embryonic stem cells were shown to survive in vivo transplantation and integrate with the host coronary circulation (7). In a phase II clinical study that evaluated the efficacy and safety of the transplantation of autologous skeletal myoblast sheets to patients with advanced heart failure due to ischemic etiology, researchers found that the cell sheet transplantation improved symptoms and prevented cardiac death in patients compared to the reference method (8).
A novel cell culture substrate to produce unidirectional mature cardiomyocyte sheets
The patterning of cell culture substrate to generate unidirectional cardiomyocyte sheets is one of the techniques used to promote maturation (9). However, popular substrates like nanofiber scaffolds and those with simple groove patterns do not promote cell-cell coordination and alignment. Here we introduce a new cell culture substrate, CellArray-Heart™, which overcomes these limitations. CellArray-Heart™ is a cell culture substrate that enables oriented cell culture simply by seeding. It features a surface microstructure with nano-sized pillars and flat areas arranged in stripes, which vary the strengths of cell adhesion and orient iPSC-CMs in one direction, to produce single flat unidirectional cell sheets. Furthermore, due to a temperature-responsive polymer coating, the cell sheets can be easily removed from the culture dishes for use in regenerative medicine applications.
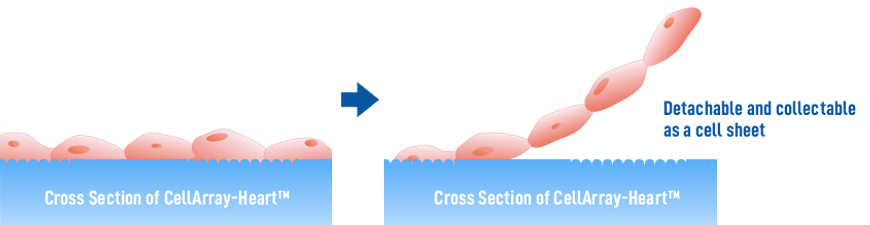
※ If it is necessary to use such a modified CellArray-Heart™, please contact us.

References
1. WHO webpage: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1
2. Mechanic, O.J., Gavin, M., and Grossman, S.A. (2022) Acute myocardial infarction (Book), Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459269/
3. Masumoto, H., & Yamashita, J. K. (2016). Human iPS Cell-Derived Cardiac Tissue Sheets: a Platform for Cardiac Regeneration. Current treatment options in cardiovascular medicine, 18(11), 65.
4. Fisher, S. A., Doree, C., Mathur, A., & Martin-Rendon, E. (2015). Meta-analysis of cell therapy trials for patients with heart failure. Circulation research, 116(8), 1361–1377.
5. Musunuru, K., Sheikh, F., Gupta, R. M., Houser, S. R., Maher, K. O., Milan, D. J., Terzic, A., Wu, J. C., & American Heart Association Council on Functional Genomics and Translational Biology; Council on Cardiovascular Disease in the Young; and Council on Cardiovascular and Stroke Nursing (2018). Induced Pluripotent Stem Cells for Cardiovascular Disease Modeling and Precision Medicine: A Scientific Statement From the American Heart Association. Circulation. Genomic and precision medicine, 11(1), e000043.
6. Ahmed, R. E., Anzai, T., Chanthra, N., & Uosaki, H. (2020). A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Frontiers in cell and developmental biology, 8, 178.
7. Stevens, K. R., Kreutziger, K. L., Dupras, S. K., Korte, F. S., Regnier, M., Muskheli, V., Nourse, M. B., Bendixen, K., Reinecke, H., & Murry, C. E. (2009). Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proceedings of the National Academy of Sciences of the United States of America, 106(39), 16568–16573.
8. Imamura, T., Kinugawa, K., Sakata, Y., Miyagawa, S., Sawa, Y., Yamazaki, K., & Ono, M. (2016). Improved clinical course of autologous skeletal myoblast sheet (TCD-51073) transplantation when compared to a propensity score-matched cardiac resynchronization therapy population. Journal of artificial organs : the official journal of the Japanese Society for Artificial Organs, 19(1), 80–86.
9. Takada, T. et al. (2022). Aligned human induced pluripotent stem cell-derived cardiac tissue improves contractile properties through promoting unidirectional and synchronous cardiomyocyte contraction. Biomaterials, 281, 121351.