Article 1
Human pluripotent stem cell-derived cardiomyocytes
– why do we need to mature them?
– why do we need to mature them?
Roshini Beenukumar, PhD

The potential of pluripotent stem cell-derived (iPSC) cardiomyocytes in applications spanning toxicological research, disease modeling, and treatment of cardiovascular diseases is immense. Among the challenges hindering their widespread adoption, the difficulty of producing mature cardiomyocytes resembling living tissue tops the list. To realize the full potential of iPSC technology in cardiac drug discovery, regenerative medicine, and cardiotoxicity testing, we need to bring together advanced technologies from various specialties – medicine, biology, engineering, and others – to tackle the issue of cardiomyocyte maturity effectively. This article discusses current maturation strategies and introduces a novel “fine stripe structure” cell culture substrate for human pluripotent stem cell-derived cardiomyocyte maturation.
Cardiovascular diseases (CVDs) claim around 17.9 million lives yearly, making them the leading cause of death worldwide (1). Among CVDs, acute myocardial infarction (AMI) is the most prevalent, causing around 3 million deaths annually (2). As myocardial infarction causes irreversible damage to the heart muscle, heart transplantation is the most effective treatment, although issues such as donor scarcity and surgical complications persist (3).
Human pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) are emerging as a promising alternative to current treatments for CVDs. Mesenchymal stem cells (MSCs) for cardiac regeneration have been shown to be safe and effective in various clinical trials. However, they don’t easily differentiate into cardiomyocytes and have poor post-transplantation retention (3,4).
Preclinical studies show that induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs), based on the iPSC technology pioneered by Yamanaka and colleagues (5), led to better cardiac outcomes when compared to MSCs (3).
Morphological and physiological immaturity of iPSC-CMs
As with any new technology, iPSC-CMs must overcome many challenges before clinical adoption. iPSC-CMs exhibit an immature phenotype in terms of structure and function. Among other insufficiencies, they show mitochondrial dysfunction, large depolarized diastolic potentials, weaker expression of myocardial-specific and gap junction proteins, and improper sarcomere formation, all of which can cause arrhythmia after transplantation (3). Therefore, achieving maturation is critical for translating iPSC-CMs into the clinic.
Several strategies have been developed to promote the maturation of iPSC-CMs. They include long-term cell culture, electric stimulation, mechanical stress, biochemical induction, and 3D tissue engineering using co-culture, ECM, and biomaterials. Although these efforts have helped, there is still much room for improvement (6).
Patterning of cell culture substrates to achieve maturation
A critical unmet need in this area is the standardization of cell culture to create an environment conducive to maturation. The physical cues experienced by the cells in cell culture must mimic the natural process of cardiomyocyte differentiation. Patterning of culture substrate is an essential tool in the maturation arsenal. However, cell-culture substrates utilizing common planar surface, fine structures and biomaterials currently used to promote maturation are not conducive to cell-cell coordination. For example, in nanofiber scaffold-based substrates, cells adhere to the fibers, and in substrates with simple groove structures, cell coordination is hindered due to the height differences.
Here we introduce a new cell culture substrate – CellArray-Heart™ – that overcomes many of the limitations of current substrates by enabling an oriented culture of iPSC-CMs simply by seeding.
Oriented cell culture to promote cardiomyocyte maturation
CellArray-Heart™ is a cell culture substrate with a surface microstructure that orients cardiomyocytes in one direction during regular cell culture. CellArray-Heart series of Cell culture dishes and multi-well plates have nano-sized pillars and flat areas arranged in stripes. This finestriped structure varies the strengths of cell adhesion, thereby orienting iPSC-CMs in one direction to produce single flat unidirectional cell sheets. Furthermore, the cell sheets can be easily removed from the culture dishes for use in regenerative medicine research applications, thanks to a temperature-responsive polymer coat.

How long do human iPSC-CMs cultured on CellArray-Heart™ maintain the orientation?
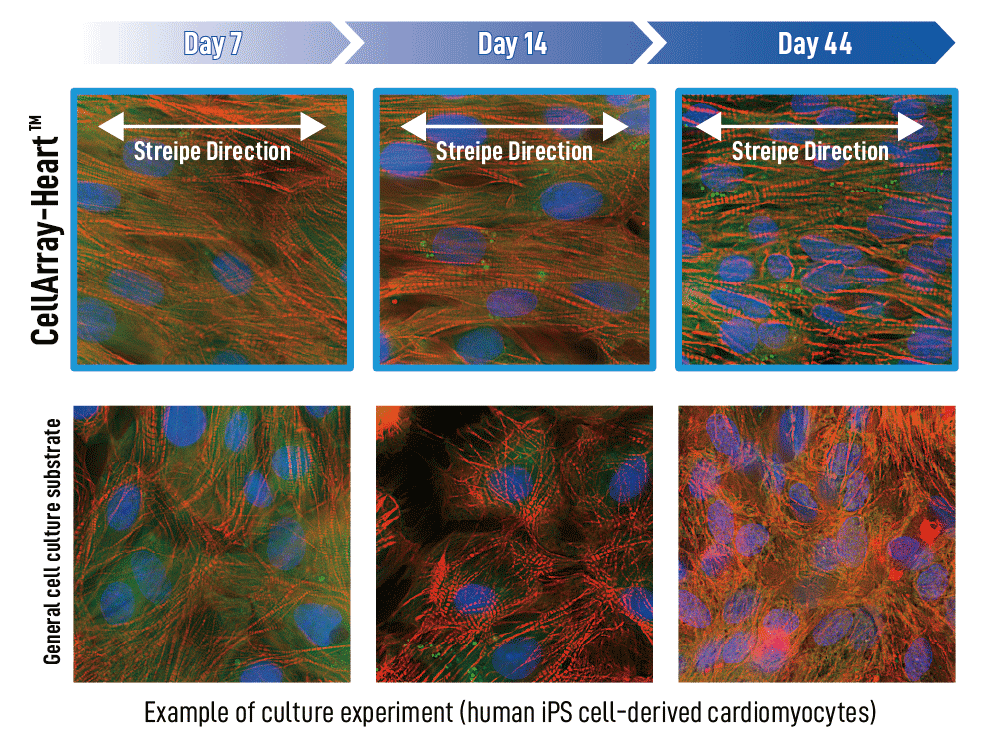
To answer this question, we performed imaging after cell culture on CellArray-Heart™ on days 7, 14, and 44 and compared it to human iPSC-CMs cultured on a regular planar cell culture substrate. The iPS cardiomyocytes cultured on CellArray-Heart™ maintained their unidirectional orientation until the 44th day. In contrast, cells cultured on the regular planar substrates showed a random orientation regardless of the number of culture days (Figure 1).
(figure 1)
How does CellArray-Heart™ affect cardiomyocyte function?
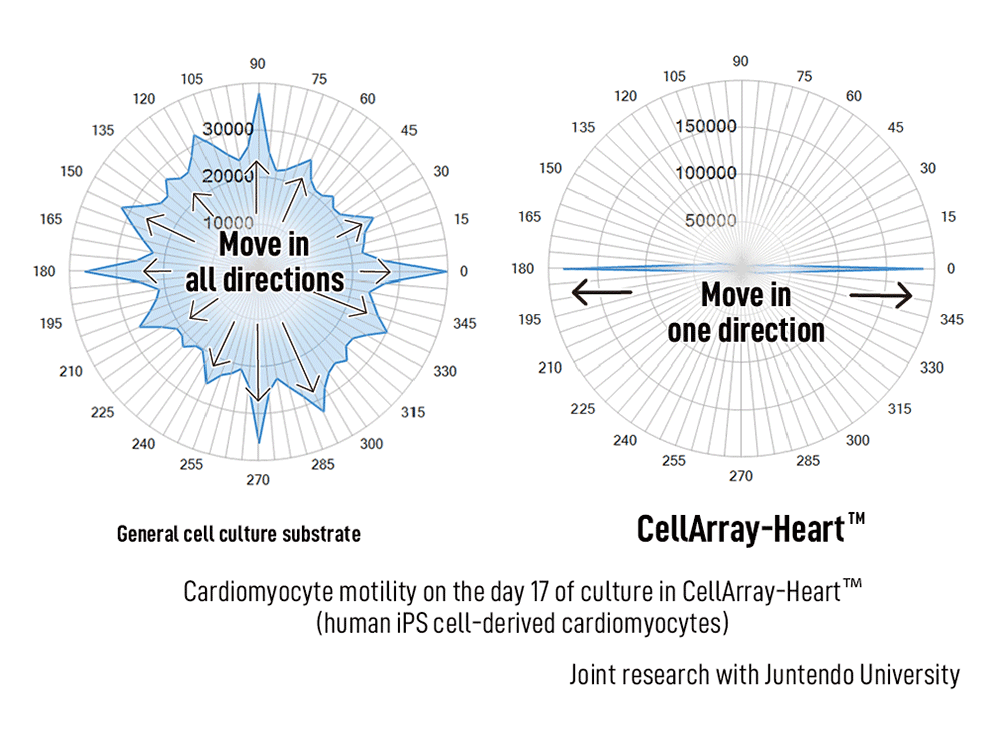
We compared cardiomyocyte motility cultured on CellArray-Heart™ with cells cultured on a regular planar substrate for 17 days using a live cell imaging system. The CellArray-Heart™-cultured cells were unidirectional in the 180° and 0° angles indicating that the cardiomyocytes contracted and relaxed in one direction, which coincided with the direction of the stripes. On the other hand, cardiomyocytes cultured on a planer substrate showed motility in all directions, indicating random directionality of contraction and relaxation (Figure 2).
(figure 2)
Obtaining fully mature iPSC-CMs resembling adult cardiomyocytes in structure, electrophysiology and function continues to be challenging for many researchers. However, with the introduction of oriented cell culture using technologies like the CellArray-Heart™, achieving adult-CM-level maturity is no longer impossible.
References
2. Mechanic, O.J., Gavin, M., and Grossman, S.A. (2022) Acute myocardial infarction (Book), Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459269/
3. Wu, P., Deng, G., Sai, X., Guo, H., Huang, H., & Zhu, P. (2021). Maturation strategies and limitations of induced pluripotent stem cell-derived cardiomyocytes. Biosci. Rep. 41(6), BSR20200833. https://doi.org/10.1042/BSR20200833
4. Trounson, A., & McDonald, C. (2015). Stem cell therapies in clinical trials: Progress and challenges. Cell stem cell, 17(1), 11–22. https://doi.org/10.1016/j.stem.2015.06.007
5. Takahashi, K. & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126 (4): 663–76. https://doi:10.1016/j.cell.2006.07.024
6. Ahmed, R. E., Anzai, T., Chanthra, N., & Uosaki, H. (2020). A brief review of current maturation methods for human induced pluripotent stem cells-derived cardiomyocytes. Front. Cell Dev. Biol. 8, 178. https://doi.org/10.3389/fcell.2020.00178
7. Abadi, P.P.S.S. et al. Engineering of mature human induced pluripotent stem cell-derived cardiomyocytes using substrates with multiscale topography. Adv. Funct. Mater. 28:1707378. https://doi.org/10.1002/adfm.201707378